Is Carbon A Gas At Room Temperature And Pressure

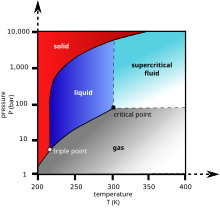
However carbon dioxide can be put under pressure to become a supercritical fluid that is a much safer dry cleaning agent than tetrachloroethylene.
Is carbon a gas at room temperature and pressure. It will be a gas. Three isotopes occur naturally 12 c and 13 c being stable while 14 c is a radionuclide. Ideal gas law calculator. Easily calculate the pressure volume temperature or quantity in moles of a gas using this combined gas law calculator boyle s law calculator charles s law calculator avogadro s law calculator and gay lussac s law calculator in one supports a variety of input metrics such as celsius fahrenheit kelvin pascals bars atmospheres and volume in both metric and.
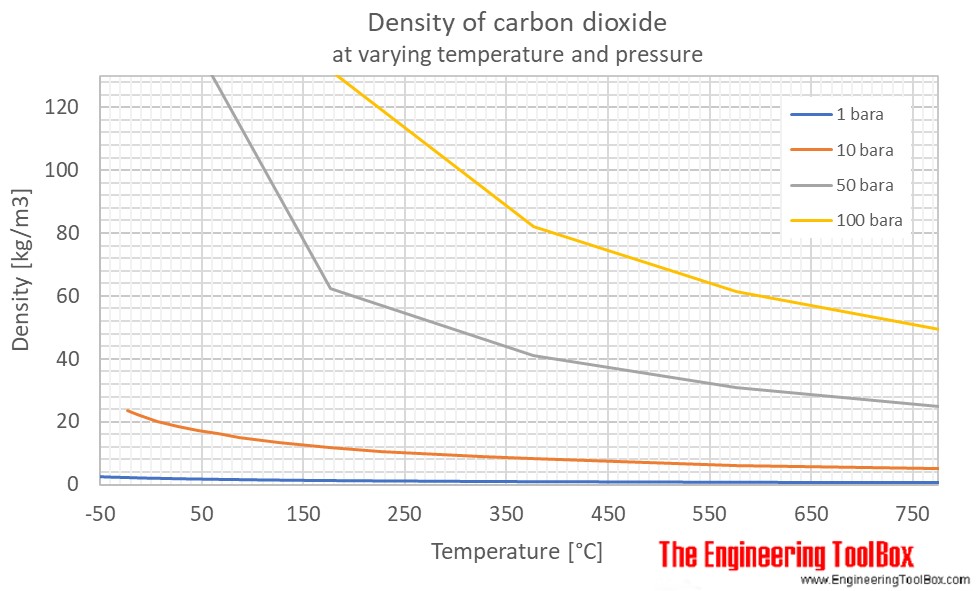
Log of carbon dioxide vapor pressure. Carbon dioxide is a colorless odorless gas at room temperature. Carbon dioxide co 2 is a gas at room temperature and pressure. It is nonmetallic and tetravalent making four electrons available to form covalent chemical bonds it belongs to group 14 of the periodic table.
Carbon is an essential building block for all forms of life and it also has important effects on climate and climate change through greenhouses gases such as carbon dioxide and carbon tetrahydride. Heat content data heat of vaporization and entropy values are relative to the liquid state at 0 c temperature and 3483 kpa pressure. At a certain pressure the density of supercritical co 2 is 0 469 g cm 3. However carbon dioxide can be put under pressure to become a supercritical fluid that is a much safer dry cleaning agent than tetrachloroethylene.
At standard temperature and pressure co2 s changes directly to co2 g. Carbon makes up only about 0 025 percent of earth s crust. At normal temperature 15 c and atmospheric pressure co 2 has a density of 1 87 kg m 3 is 1 5 times heavier than air and spreads along the ground collecting in low lying areas such as pits and cellars. It is a colourless and odourless gas with a slightly pungent.
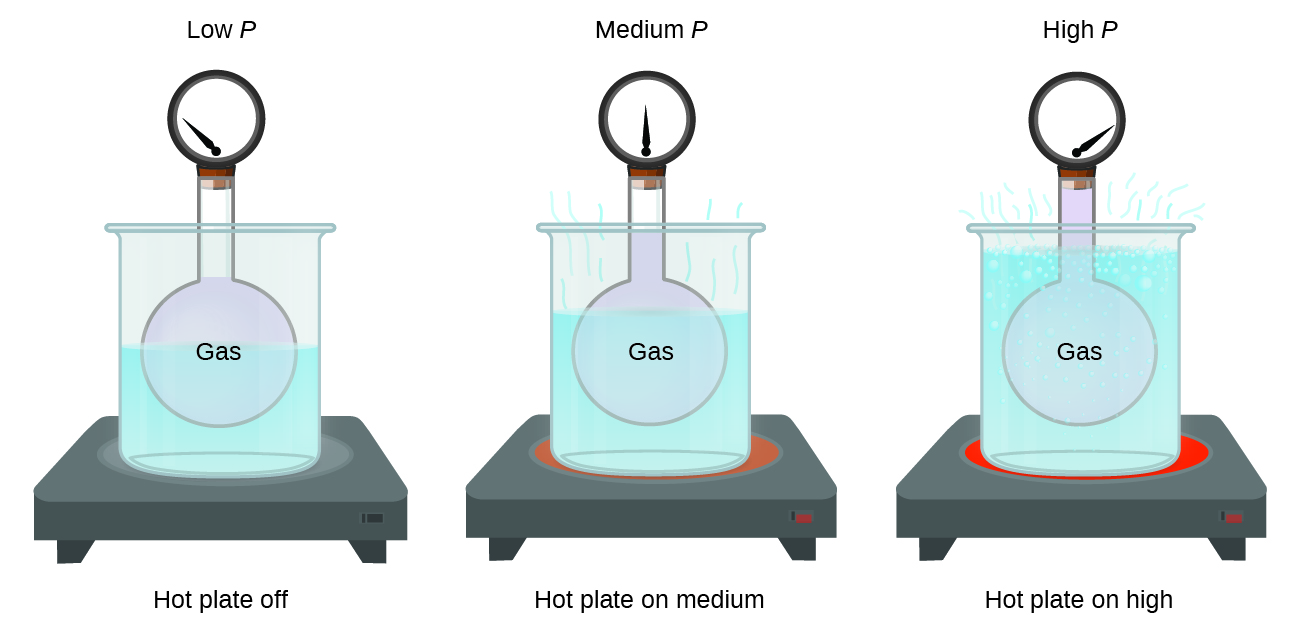
As the pressure on a gas confined above a liquid increases the solubility of the gas in the liquid. Gas properties std enthalpy change. Carbon forms molecular compounds with some elements from group 16. Two of these compounds are carbon dioxide co2 and carbon disulfide cs2.
At a certain pressure the density of supercritical co2 is 0 469 g cm3. At room temperature the solubility of which solute in water would be most affected by a change in pressure. Carbon dioxide co2 is a gas at room temperature and pressure. To convert heat values to joules per mole values multiply by 44 095 g mol.
Carbon disulfide is formed by a direct reaction of carbon and sulfur.