Iso Medical Device Labeling Standards

It also lists symbols that satisfy the requirements of this document.
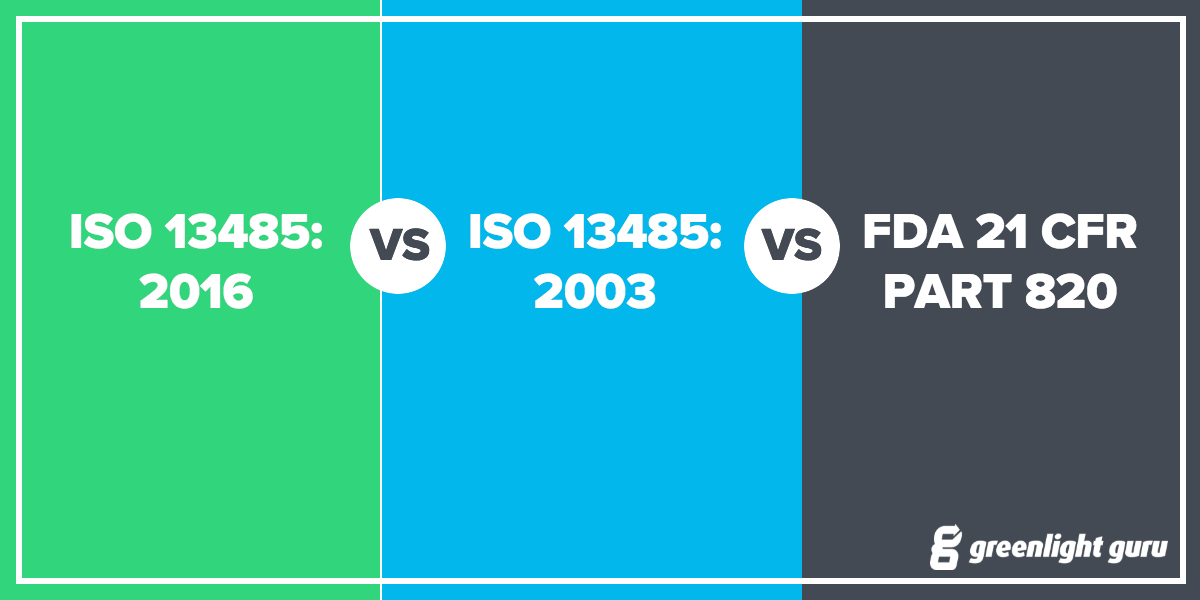
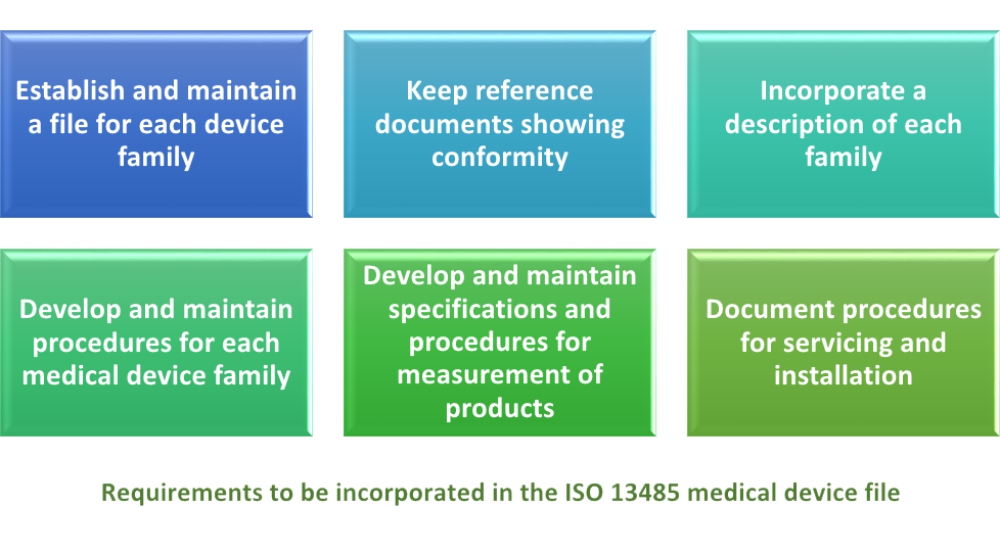
Iso medical device labeling standards. The iso 13485 standard was entitled quality systems medical devices. This article explains the method starting with standards from the international organization for standardization iso adopted and recognized in various regulatory systems. An interlaboratory comparison of analytical methods for ethylene oxide pb 86. It represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices this standard supersedes earlier documents such as en 46001 1993.
Wherever requirements are specified as applying to medical devices the requirements apply equally to associated services as supplied by the organization. Iso 13485 medical devices quality management systems requirements for regulatory purposes is an international organization for standardization iso standard published for the first time in 1996. Sign up to our newsletter for the latest news views and product information. P one common source of misunderstanding in the medical device industry is the method the various national regulatory systems use to identify standards.
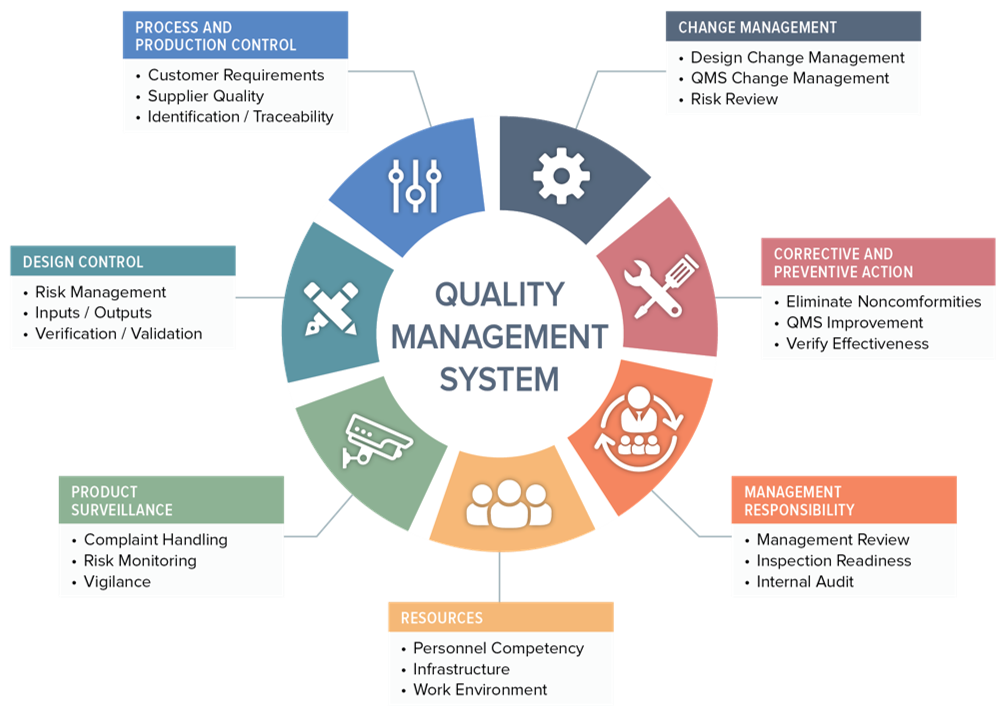
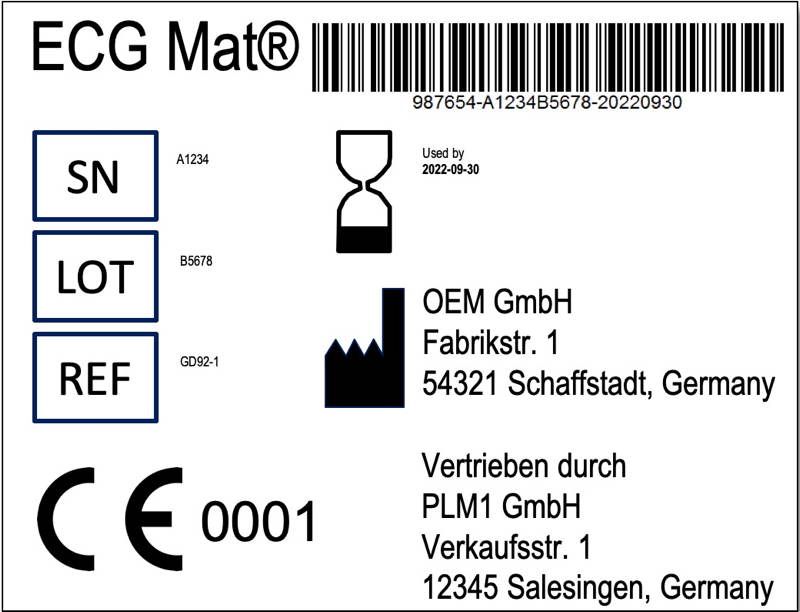
Requirements of iso 13485 2016 are applicable to organizations regardless of their size and regardless of their type except where explicitly stated. In iec 60601 1 labeling is deemed a critical component of a medical device 1 the standard provides comprehensive requirements for medical device marking and labeling. Medical device labeling is considered as important as classifying a product or creating an insulation diagram. Labeling regulatory requirements for medical devices gpo 017 012 00327 3 2 75 pb 86 184348 as 11 95.
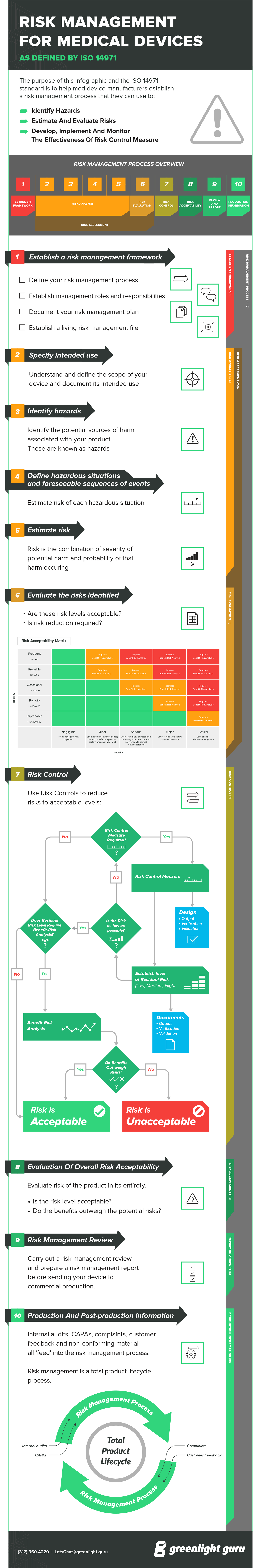
The iso 9001 standard was entitled quality systems model for quality assurance in design development production installation and servicing. The article uses iso 13485 2003 and iso 14971 2007 as illustrations p. The primary standards included international organization for standards iso 9001 1994 and 13485 1996. Iso 13485 is the best internationally accepted model a medical device organization can implement to help demonstrate compliance to laws and regulations of the medical device industry.
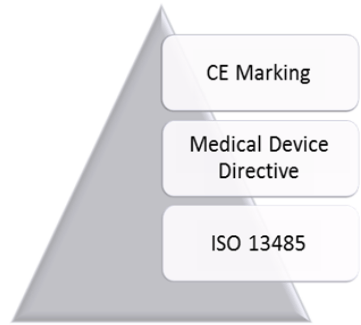
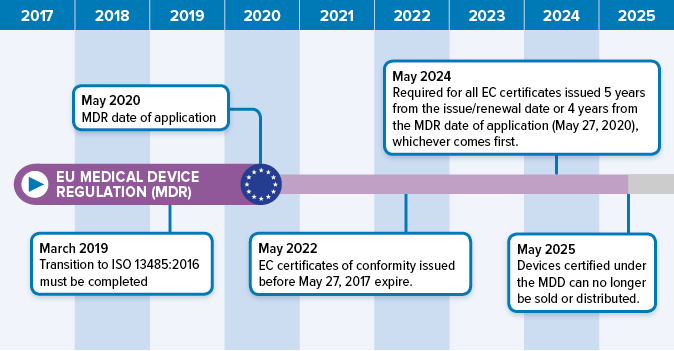
Iso 13485 is the quality management system standard accepted as the basis for ce marking medical devices under european directives and regulations.